Global Respiratory Inhaler Devices Market Outlook 2026–2036: Smart & Low-Carbon Innovation Fuels 6.0% CAGR
Global market to reach USD 68.1 Billion by 2036, driven by rising COPD, asthma cases and low-carbon, AI-enabled inhalers.
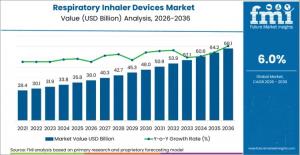
NEWARK, DE, UNITED STATES, February 13, 2026 /EINPresswire.com/ -- The global Respiratory Inhaler Devices Market is projected to reach USD 68.1 billion by 2036, rising from USD 38.0 billion in 2026 at a CAGR of 6.0%, according to Future Market Insights (FMI). Growth is structurally anchored in the rising prevalence of asthma and chronic obstructive pulmonary disease (COPD), alongside regulatory-driven transitions toward low-carbon propellants and digitally connected inhaler technologies.
Rising Global Respiratory Disease Burden Drives Device Demand
Chronic respiratory diseases affect more than 545 million people globally, with asthma and COPD accounting for the majority of inhaler prescriptions. Increasing air pollution exposure, aging populations, and urbanization trends are accelerating disease incidence across both developed and emerging markets.
In the United States alone, over 25 million individuals live with asthma, while approximately 16 million are diagnosed with COPD. Similar patterns are visible across Europe and Asia-Pacific, where environmental risk factors and demographic shifts are expanding the patient base for long-term maintenance therapies.
Request For Sample Report | Customize Report | Purchase Full Report–
https://www.futuremarketinsights.com/reports/sample/rep-gb-493
As a result, pharmaceutical companies and medical device manufacturers are scaling investments in:
- Drug-device combination innovations
- Breath-actuated and precision-dose inhalers
- Expanded generic inhaler portfolios
- Digital adherence monitoring systems
Sustainability Mandates Reshape pMDI Portfolios
One of the most transformative structural shifts in the industry is the transition away from high-global-warming-potential propellants in pressurized metered-dose inhalers (pMDIs).
In May 2025, AstraZeneca plc received approval from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for its low-carbon Trixeo Aerosphere, replacing conventional hydrofluorocarbon propellants with HFO-1234ze(E), reducing global warming potential by 99.9%.
Similarly, Chiesi Group completed its Phase III TRECOS study in September 2025, validating its HFA-152a-based carbon-minimal inhaler platform. This milestone forms part of a EUR 400 million sustainability investment program.
These regulatory and clinical breakthroughs are expected to:
- Accelerate industry-wide reformulation timelines
- Increase R&D spending on low-GWP propellants
- Differentiate premium branded inhalers
- Strengthen ESG positioning across pharmaceutical portfolios
Generic Pipeline Expansion Pressures Pricing
While innovation fuels premium growth, the expanding generic inhaler pipeline is reshaping pricing dynamics—particularly in the United States.
In December 2025, Amneal Pharmaceuticals received U.S. FDA approval for its generic version of PROAIR HFA, following earlier approval of a QVAR equivalent. This trend reflects a broader movement toward complex generic inhalers that enhance affordability and widen patient access.
FMI analysis indicates the market is evolving into a bifurcated structure:
Premium pricing for next-generation low-carbon and smart inhalers
Volume-driven growth in cost-effective generic MDIs and DPIs
The United States continues to hold a significant market share by value, supported by high per-capita respiratory drug spending and strong reimbursement frameworks.
Product Trends: DPIs and MDIs Lead Adoption
Dry Powder Inhalers (DPIs) and Metered Dose Inhalers (MDIs) remain the dominant product segments.
Dry Powder Inhalers (DPIs) are increasingly preferred due to:
- Propellant-free drug delivery
- Breath-actuated mechanisms
- Lower environmental footprint
- Improved patient compliance
Multi-dose DPIs with integrated dose counters and digital tracking are gaining traction, particularly in long-term asthma and COPD management.
Metered Dose Inhalers (MDIs) continue to dominate acute care settings, especially for rescue therapies such as short-acting beta-agonists (SABAs). Their compact design, rapid bronchodilation, and cost efficiency ensure sustained demand despite environmental scrutiny.
Emerging breath-actuated MDIs and eco-friendly propellant alternatives are helping sustain this segment’s relevance.
Digital Inhalers Redefine Adherence & Remote Monitoring
Digitally operated inhaler systems represent one of the fastest-evolving segments within the market. Bluetooth-enabled and AI-integrated inhalers now offer:
- Real-time dose tracking
- Inhalation technique analysis
- Smartphone app integration
- Predictive alerts for exacerbations
Integration with electronic health records (EHRs) allows clinicians to monitor longitudinal adherence patterns and personalize treatment regimens.
However, adoption remains constrained by:
- Higher upfront device costs
- Limited insurance coverage in certain markets
- Data privacy and cybersecurity considerations
Despite these challenges, digital inhalers are expected to become a core component of chronic respiratory disease management by 2036.
Regional Outlook: North America Leads, Asia-Pacific Accelerates
North America remains the largest regional market, driven by advanced healthcare infrastructure, high disease awareness, and rapid uptake of smart inhalers.
Europe is witnessing strong regulatory momentum toward low-carbon inhalers, with environmental compliance shaping product innovation strategies.
Asia-Pacific is projected to register the highest CAGR, fueled by rising air pollution levels in China and India, expanding healthcare access, and growth in telemedicine platforms.
Governments across emerging economies are investing in respiratory care initiatives, improving rural access to inhalation therapies and promoting affordable generic options.
Competitive Landscape: Innovation & Portfolio Diversification
Market leadership is concentrated among established pharmaceutical giants. Key players include:
- GlaxoSmithKline plc
- AstraZeneca plc
- Boehringer Ingelheim International GmbH
- Teva Pharmaceutical Industries Ltd.
- Novartis AG
Collectively, these firms are advancing smart inhalers, breath-actuated platforms, and AI-driven respiratory analytics while navigating regulatory transitions toward sustainable propellant systems.
Future Outlook: 2026–2036 Innovation Wave
Between 2026 and 2036, the respiratory inhaler devices industry is expected to evolve beyond conventional drug delivery into precision pulmonary platforms.
Key anticipated advancements include:
- AI-assisted dose optimization
- Inhaler-integrated lung biometrics
- Biodegradable inhaler materials
- Regenerative and nano-medicine inhalation therapies
- Predictive analytics for early exacerbation detection
Sustainability, personalization, and digital integration will define competitive advantage over the next decade.
Related Reports:
Liver Disease Diagnostics Market – https://www.futuremarketinsights.com/reports/liver-disease-diagnostics-market
Medical Waste Liquid Collection Device Market – https://www.futuremarketinsights.com/reports/medical-waste-liquid-collection-device-market
Biocatalytic APIs Market – https://www.futuremarketinsights.com/reports/biocatalytic-apis-market
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
Why FMI: https://www.futuremarketinsights.com/why-fmi
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.